Recombinant Proteins & Antibodies
Therapeutic proteins | mAbs and BsAbs | Recombinant enzymes | Biosimilars
Therapeutic proteins | mAbs and BsAbs | Recombinant enzymes | Biosimilars
We are an end-to-end recombinant protein CDMO with a track record in developing and manufacturing a wide variety of protein and antibody types, from small scale up to 1,000 L SUB scale. Our experience spans a wide variety of different classes of biologics, such as recombinant proteins, novel monoclonal antibodies, bispecific antibodies, and biosimilars.
HALIX manufactures recombinant proteins and antibodies in our protein production areas comprised of several GMP class C cleanrooms for cell culturing, upstream and downstream processing, and Class B cleanrooms for fill and finish. Our technology platform and in-depth know how ensures smooth scale up from lab to industrial scale , while our transparent and agile project management ensures minimum risks.

Diverse range of cell lines
e.g. HEK293, CHO, PER.C6, HeLa

Extensive expertise in cell banking
including research cell banks, master cell banks, working cell banks, for both adherent and suspension cell cultures

Complete manufacturing cycle in house
including USP (bioreactors, roller bottles, cell factories), DSP (chromatography, filtration, TFF), and DP (aseptic fill and finish in vials, lyophilization)

Comprehensive GMP experience
with a wide range of services including TOX and Tech batches, placebo, clinical and commercial supply
Exceptional speed and adaptability for clinical trial material
From cell banks to final product release. QA & QP guidance during complete process.
Financial transparency, clear communication on progress throughout the project Ability for fast scale-up to a 50L bioreactor in GMP.
Delivery of first clinical batch within 7 months, on time for clinical trial. Ongoing partnership over the last 5 years.
“In HALIX we found the ideal partner:
experience in transient transfection, modern facilities, strong leadership and a laser focus on delivery to bring NVG-111 from contract signature to vials of clinical drug in less than seven months.“
Kieran O’Donovan, COO and CTO at Novalgen
Developing and manufacturing recombinant protein products comes with its own unique set of difficulties and risks. Whether you are a small biotech or a large pharma, open communication between your organization and the recombinant protein CDMO partner is key to achieving success.
At HALIX we commit to full transparency and alignment with you every step of the way. Our project management approach is built on a foundation of open communication and agility, with short and direct communication channels, allowing us to quickly adapt and respond to any obstacles that may arise.
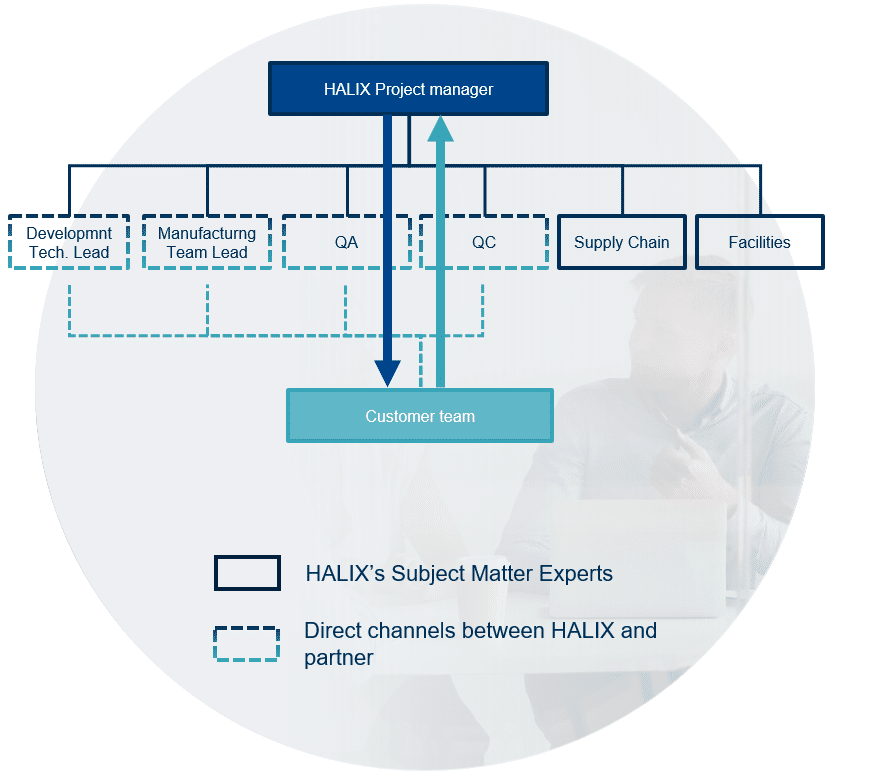
As an end-to-end recombinant protein CDMO, we work closely with our partners to create tailor-made process analytical development at a scale required for individual projects, produce drug substance and drug product with a flexible approach for seamless customer process transfer and perform in-house testing ensuring all products meet strict quality standards.
We support your product development with scalable and robust small scale production, analytical development, and upscaling services.
We have a solid track record in the production of recombinant proteins and antibodies, from tech and TOX batches to commercial scale, under cGMP.