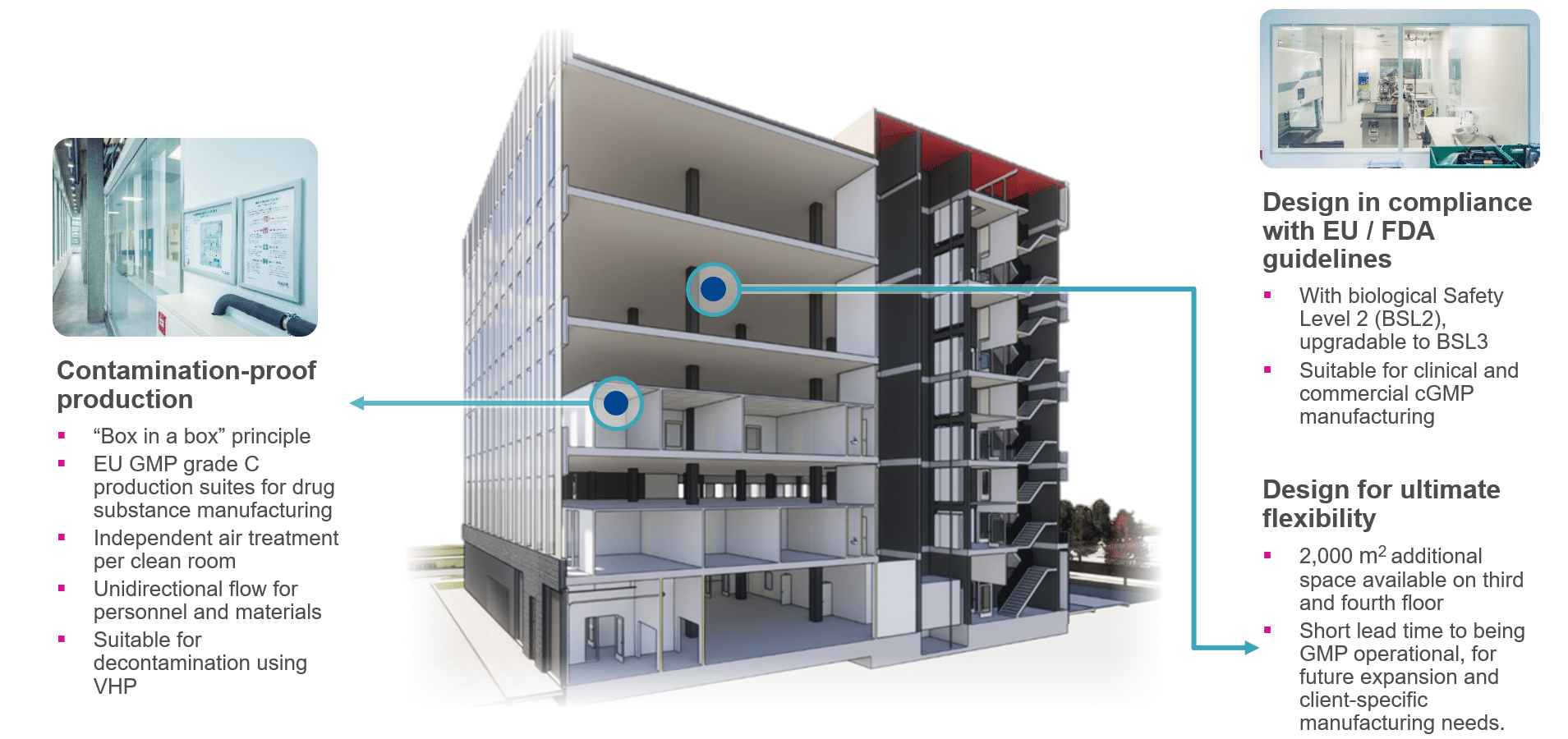
Our state-of-the-art facility for the development and production of biopharmaceutical drug substances and drug products is operational in the Leiden Bio Science Park, the Netherlands since 2019. Designed in compliance to the latest GMP standards, HALIX produces biologics on four floors with 6,700 m2 development and production area.
cGMP production services and capabilities
Our fully licensed production facility in the Netherlands allows for flexible and scalable contract development and cGMP production from preclinical to commercial scale products. Our production capabilities cover the whole manufacturing spectrum and include:
- Strong experience in adherent and suspension cell lines
- Upstream processing, from small scale up to 1,000 L scale
- Purification using depth filtration, tangential flow filtration (TFF) and chromatography systems
- Single use disposable equipment
- Large scale manufacturing of BSL1, BSL2 and BSL3 viruses
- Development using Quality by Design (QbD) principles
- Analytical method development and optimization
- Analytical method qualification and QC method validation
- Early stage process development and Design of Experiment (DoE)
- Process optimization and process scale-up
- scale down models and viral clearance studies
- Early and late stage process
- Engineering toxicology batches and GLP TOX study batches
- GMP manufacturing of clinical and commercial Drug Substance and Drug Product, including cell banks and virus seeds
- QC release testing
- Process Performance Qualification (PPQ) and supporting studies
- EU GMP grade C production suites for drug substance manufacturing
- Independent air treatment per clean room
- Unidirectional flow for personnel and materials
- Suitable for decontamination using VHP
- Pharmaceutical documentation system
- Quality management system
- GMP and commercial manufacturing certificate and regular GMP inspections
- Comprehensive quality assurance and Quality Management System (QMS), in compliance with EU GMP, FDA, ICH and other industry standards and guidelines
- Short communication channels by providing direct access to key HALIX project team members
- Project supervision by Sr. management of the customer & scientifically qualified Sr. management from HALIX
- Regular project team meetings ensure progress, alignment, mitigate risks and proactively manage critical activities
- Specialized and interdisciplinary teams fully committed to the project’s success
- One project manager as single use of contact